In the course of TIGHAR’s effort to solve the mystery of what happened to Amelia Earhart, only a few artifacts have generated as much curiosity and controversy as the five glass shards cataloged by TIGHAR as 2-9-S-1a through 2-9-S-1e, collectively named 2-9-S-1. Together, these shards form a nearly complete jar, identified as an ointment pot. Initial comparative studies suggested that it might represent a container of Dr. Berry’s Freckle Ointment, a skin-lightening ointment that Amelia Earhart, who had freckles, might have used. However, the relevant comparative examples we were able to find in contemporary collections were made of opaque “milk glass,” while 2-9-S-1 is made of colorless, translucent glass. The presumed reason for using fully opaque glass was to protect the ointment, whose active, and toxic, ingredient was mercury, from ultraviolet (UV) exposure.
In the years since 2-9-S-1 was unearthed at the Seven Site on Nikumaroro Island in July 2010, we have made a concerted attempt to determine its age, character, original contents, and historical context. We have learned much, and the research is ongoing. With the understanding that total knowledge is an ideal one may seek but never fully achieve, we feel the time is ripe for a summary of our findings:
- We found mercury on the glass of 2-9-S-1: Laboratory tests indicate 2-9-S-1 had mercury adhering to its interior surface. No mercury was found adhering to its exterior surface.1 The mercury is likely to have been compounded in a form which was not volatile and therefore persisted on the artifact’s surface for decades. Non-volatile ammoniated mercury was the active ingredient in freckle ointments. Dr. Berry’s Freckle Ointment sold in this style of jar from 1908 to at least 1933.
- We did not find mercury on nearby glass: A laboratory tested another glass shard from a World War II vintage Coca-Cola bottle recovered a few meters from where 2-9-S-1 was found. This shard did not have any mercury adhering to its interior or exterior surfaces.
- We verified that mercury in freckle ointment will adhere to glass: A laboratory tested a verified jar of Dr. Berry’s Freckle Ointment, in the same style of jar as 2-9-S-1 (but made of milk glass). This verified Dr. Berry jar was emptied and rinsed thoroughly of its original contents before testing. Mercury was found adhering at high levels to the interior surface of its glass. Mercury was not found adhering to its exterior surface. Mercury was also found in significant quantity in the freckle ointment itself.
-
 |
| 2-9-S-1 is on the left. TIGHAR Photo. |
We verified that not all contents of similar jars contained mercury: A laboratory tested a verified jar of Burnham Kalos Skin Rejuvenator, which also sold in the same style of jar as 2-9-S-1. This verified Burnham jar was emptied and rinsed thoroughly of its original contents before testing. No mercury was found adhering either to its interior or exterior surfaces. No mercury was found in the ointment itself.
- We verified that 2-9-S-1 was most likely manufactured in the 1930s: Chemical analysis shows that the composition of the glass in 2-9-S-1 was unusual. It was not a run-of-the-mill soda lime glass of the type used for disposable jars. A complete assay of the elements in 2-9-S-1 was linked to a unique Hazel-Atlas glass manufacture patent from 1936, indicating the possibility 2-9-S-1 may have been manufactured in the years just prior to Amelia Earhart’s world flight.
|
|
|
2-9-S-1 is a 2-ounce, tapered, footed, continuous threaded jar. Its glass is not brittle, but rather of durable, dense construction, measuring 6 to 7 mm in thickness on the sides. It would not easily shatter if dropped from a standing height. The glass is colorless but not transparent; rather, it is translucent in appearance. 2-9-S-1 has a lustrous brilliance when lighted, unlike a common soda lime glass. Double lipped at its base, 2-9-S-1 has flowing art deco lines and a feminine appearance.

Figure 1. The brilliance of the glass from a base shard of 2-9-S-1 is evident from this photograph. TIGHAR photo by Joe Cerniglia. |
|
|
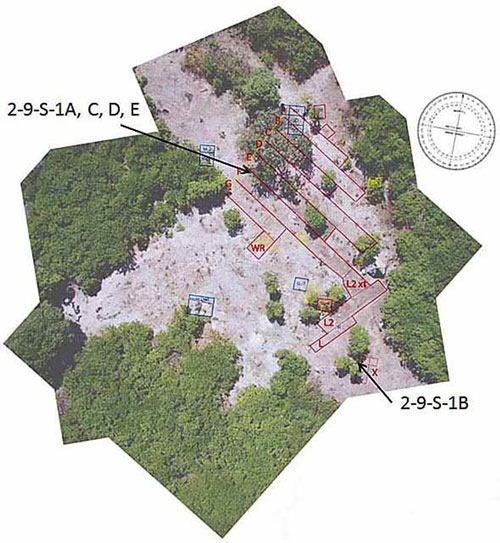 |
| Figure 2. Locations of shards from 2-9-S-1. TIGHAR photo. |
2-9-S-1 was found at the Seven Site, named for a natural clearing shaped like a numeral “7” in the dense underbrush that grows on the island. The site lies on the southeast corner of Nikumaroro, an equatorial island in the Republic of Kiribati in the Phoenix Group, located in the south central Pacific Ocean. The base was broken into two roughly equal fragments, and the sides were broken into three additional fragments, comprising, when reassembled, a nearly complete jar. Four of the five shards, including about half of the jar’s body and both halves of its base, were found in a loose cluster near the north end of the site. The fifth shard, 2-9-S-1b, comprising the remaining half of the body, was found about thirty meters southeast of the others. All were found in the top 10 centimeters of the coral rubble that makes up the site. Other glass artifacts recovered from the site include a Coca-Cola bottle, a beer bottle, three probable lotion or ointment bottles (Mennen Skin Bracer or Mennen Baby Oil, St. Joseph Nerve & Bone Liniment, Campana Italian Balm), and an octagonal bottle that probably represents a Coast Guard salt shaker. All were fragmentary; 2-9-S-1 is among the most complete glass containers yet found at the Seven Site.
While other glass containers at the site may have been used as targets of shooting exercises by personnel from the nearby 1944-46 U.S. Coast Guard Station, 2-9-S-1 shows no clear evidence of having been struck by bullets.
Dr. Geoffrey Cunnar, a specialist in use wear patterns on stone and glass artifacts, examined two of the shards. He reported that 2-9-S-1b, a side shard from the jar, showed what he interpreted as fair to good signs that it had been used as a tool for scraping soft material such as plants or wood. He reported that 2-9-S-1a, another side shard from the jar, showed what he interpreted as fair to good signs that it had been used to scrape medium-hard material.2
|
Earliest History of 2-9-S-1 |
The earliest documented drawing we have found of 2-9-S-1’s general design was for an “Ointment Pot” listed on page 29 of the 1896 Whitall Tatum & Company catalog. The design differs slightly in that it has a single-lipped base rather than the double-lipped base found on 2-9-S-1. The Whitall Tatum jar is also squatter than 2-9-S-1.

Figure 3. Whitall Tatum & Company advertisement for earliest jar in general style of 2-9-S-1.
By at least 1910, any patent for this jar would have expired, making the design usable by any other company that wanted to use it. |
When Was 2-9-S-1 Manufactured? |
1. The Hazel-Atlas Mark
The base underside of 2-9-S-1 is embossed with the manufacturer’s mark, H over A, of the Hazel-Atlas Glass Company, which was in business from 1902 to 1957 and was headquartered in Wheeling, West Virginia.3

Figure 4. Close-up of Hazel-Atlas logo on underside of base of 2-9-S-1. TIGHAR photo.
Hazel-Atlas illustrated these jars in their early 20th century catalogs, under the heading of ointment pots.
Based on research of period advertisements, 1918 is our best estimate for the earliest year in which the Hazel-Atlas Glass Company produced ointment pots in the style of 2-9-S-1. Roughly 22 years had elapsed between the Whitall Tatum Company jar in 1896 and the year in which Hazel-Atlas apparently began manufacturing jars in this style. The design of 2-9-S-1 was, therefore, already somewhat old-fashioned when Hazel-Atlas started using it. Based on advertisements we have traced, the style does not seem to have been in common use in the marketplace. Only a handful of products appear to have been contained in this style of jar.
 |
 |
| Figure 5. Complete view of base underside, with the Hazel-Atlas manufacturer’s mark as it appears on 2-9-S-1. TIGHAR photo. |
Figure 6. Hazel-Atlas advertisement in the 1918 (Vol. 48) of National Druggist magazine. The red arrow has been added for emphasis and is not part of the original copy. |
2. The Hazel-Atlas Trademark Application Provides a No-Earlier-Than Date for 2-9-S-1
 Hazel-Atlas applied for a trademark for its “H over A” logo, which appears on the base underside of 2-9-S-1, on February 2, 1924. Hazel-Atlas applied for a trademark for its “H over A” logo, which appears on the base underside of 2-9-S-1, on February 2, 1924.
The company stated in its application: “The trademark has been continuously used and applied to said goods in applicant’s business since July 23, 1923.” Although there has been much discussion about whether this date represents the true first use of the mark on all Hazel-Atlas goods, we have no reason to think that Hazel-Atlas used their mark prior to the date they claimed in this application. We have searched and found no earlier claim for the Hazel-Atlas trademark. Claiming the earliest possible first use date was, and still is, the best way to secure senior rights to a mark; therefore, it made sense for the Hazel-Atlas Company to claim the earliest possible date of first use of the mark. The fact that Hazel-Atlas did not mention jars specifically in its application could well lead to the conclusion that jars were embossed with the Hazel-Atlas mark after 1923, not earlier.4 There is very good documentation for our belief that 2-9-S-1, therefore, was manufactured on or after July 23, 1923, and no earlier than this date.

Figure 7. Hazel-Atlas trademark application for the H over A logo, with earliest use date of July 23, 1923.
3. Linking 2-9-S-1’s Special Glass Chemistry with a Unique Glass Formula from the 1930s
 Analysis of the elements in the glass of 2-9-S-1, as compared with the elements in a clear jar in the same style, revealed an unusual chemistry, most notably a high concentration of barium and magnesium.5 (See Clear Jar Report.) Analysis of the elements in the glass of 2-9-S-1, as compared with the elements in a clear jar in the same style, revealed an unusual chemistry, most notably a high concentration of barium and magnesium.5 (See Clear Jar Report.)
Barium was, and is, employed in glass manufacture to add luster or brilliance to glass, and also to raise the refractive index.6 Museum glass, salt shakers, shot glasses, and microscopic slides are often high in barium.
A high refractive index offers protection from ultraviolet light and would have been essential in an ointment jar, since many compounds in ointments, mercury among them, degrade in ultraviolet light from the sun. The photo-reactivity of these compounds was well understood long before the earliest proposed manufacture date of 2-9-S-1 in 1923.7 2-9-S-1 is not fully opaque, and therefore would admit some sunlight. However, due to its high barium content, it would still protect its contents from ultraviolet light. 2-9-S-1 was, therefore, entirely suitable as a container in which to store an ointment.
The barium found in the glass of 2-9-S-1, measured at .74% weight, seemed to serve a very useful and readily explainable function. We were puzzled, however, by the high levels of magnesium in the glass of 2-9-S-1, measured at 4.3% weight. Levels this high are seldom found in ordinary container glass, and are even much higher than levels found in window (float) glass.
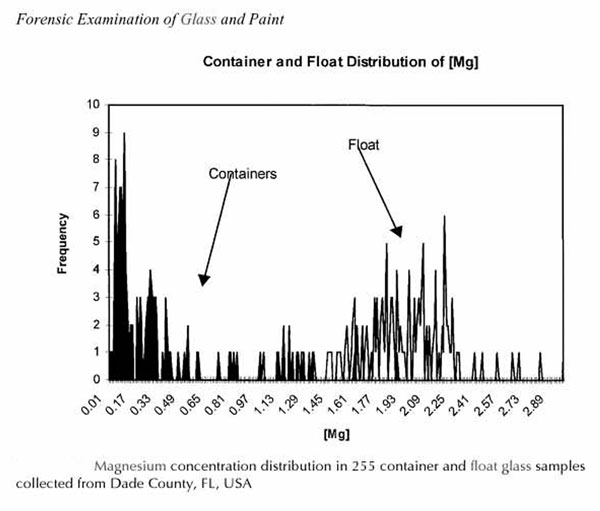
Figure 8. Comparison of magnesium percentage weight in common container glass (dark lines) and float glass (light lines).8
To understand why 2-9-S-1 has high levels of magnesium, it is necessary to explain the chemistry used in 2-9-S-1’s manufacture, and the manufacture of glass more generally. Barium is not added to a glass batch by itself, but rather it is often added as part of a compound. Barium oxide is commonly used, but glassmakers historically also used barium sulfate. Barium sulfate serves a dual purpose in glass manufacture. The barium in the compound, known as a trace ingredient, is used to increase brilliance; the sulfate serves as a fining agent to facilitate the removal of bubbles from the glass mixture. The removal of the bubbles is highly desirable in creating a blemish-free glass. However, sulfate, once added to a glass batch, also inhibits the silica from combining with the soda lime, which, in turn, prevents the glass batch from flowing, which makes it impossible to make the glass. To derive the full benefit of the bubble-releasing characteristics of the sulfate, while minimizing its potential to prevent proper mixing of the batch, the sulfate must be reduced after it has been added.
To reduce the sulfate, it is necessary to add a reducing agent. Magnesium, it was discovered, was one of a number of metals that are useful in the reduction process. Magnesium can reduce sulfates, as well as other impurities, such as iron. A search for relevant glass patents that used barium for brilliance and magnesium for reducing sulfates during the first half of the 20th century revealed only two patents.
 The first patent we found, U.S. Patent Number 2,218,334, was submitted on August 28, 1937. This patent relates to the use of a fluorspar-barite ore as a “fluxing and fining agent,” with a corresponding use of magnesium chloride to reduce iron impurities, as well as to reduce sulfates, present in the barite of the glass mixture. (See patent documentation.) The first patent we found, U.S. Patent Number 2,218,334, was submitted on August 28, 1937. This patent relates to the use of a fluorspar-barite ore as a “fluxing and fining agent,” with a corresponding use of magnesium chloride to reduce iron impurities, as well as to reduce sulfates, present in the barite of the glass mixture. (See patent documentation.)
 In order to show that this patent corresponded with the glass chemistry of 2-9-S-1, however, we needed to show that fluorspar, in the form of the element fluorine, was contained in 2-9-S-1’s glass chemistry. Due to the fact that a specialized test, known as X-ray fluorescence, showed no fluorine present in the glass, we were able to eliminate this patent from consideration as representing the chemistry of 2-9-S-1. (See Fluorine Report.) In order to show that this patent corresponded with the glass chemistry of 2-9-S-1, however, we needed to show that fluorspar, in the form of the element fluorine, was contained in 2-9-S-1’s glass chemistry. Due to the fact that a specialized test, known as X-ray fluorescence, showed no fluorine present in the glass, we were able to eliminate this patent from consideration as representing the chemistry of 2-9-S-1. (See Fluorine Report.)
 A second patent proved to be far more relevant. The Hazel-Atlas Glass Company submitted U.S. Patent Number 2,113,195 on March 6, 1936 for the use of magnesium as a reducing agent for barium sulfate. (See patent documentation.) This patent relates specifically to barium and magnesium, both found at high levels in the glass of 2-9-S-1, and it has no special requirement for fluorspar in the glass mix. A second patent proved to be far more relevant. The Hazel-Atlas Glass Company submitted U.S. Patent Number 2,113,195 on March 6, 1936 for the use of magnesium as a reducing agent for barium sulfate. (See patent documentation.) This patent relates specifically to barium and magnesium, both found at high levels in the glass of 2-9-S-1, and it has no special requirement for fluorspar in the glass mix.
Francis Flint, chief chemist at the Hazel-Atlas Glass Company and author of the patent, stated that the amount of magnesium used was not a critical concern: “I am able to do this without any unusual accuracy or care in determining an exact amount of the reducing agent to be employed in a particular batch…” Flint’s comment appears to explain why the magnesium level is so high in 2-9-S-1.
Hazel-Atlas had experimented with carbon as a reducing agent; however, reduction of the sulfates with carbon had yielded unsatisfying results, since carbon canceled out the beneficial fining properties of the sulfate, causing bubbles (“seed, gall or stones”) to form. Thus, the patent details a way to keep both the high luster and ultraviolet protection afforded by the barium while allowing the sulfate to do its work in fining the glass mixture. By reducing the sulfate with magnesium once the fining stage is complete, the silica is allowed to combine effectively with the soda-lime.
This patent, therefore, outlines an unusual and highly complex glass chemistry, which 2-9-S-1 shares, which would most likely not have been used in routine glass production. The language of the patent itself states that Hazel-Atlas enhanced its glass formulas in the face of tighter industry standards that were a recent phenomenon in 1936. The patent states in its second paragraph: “… in recent years the requirements with respect to container glass and the like have become more and more rigid.” These higher standards pertained to the “color and brilliance” of glass, which up until that point were said to “have not been very severe.”
The language and the date on the patent, as well as that patent’s superb correlation with the chemistry of the glass in 2-9-S-1 and with the Hazel-Atlas Glass Company, whose mark 2-9-S-1 shares, would therefore appear to indicate 2-9-S-1 was far more probably manufactured in the 1930s than in the 1920s. On the other hand, this date by no means rules out the possibility that 2-9-S-1 was manufactured after 1936.
|
| Searching for Siblings: Historical Examples |
To date, the complete list of U.S. commercial products we have identified that were packaged in the same style jar as the one found on Nikumaroro are:
Dr. Berry’s Freckle Ointment9
Dr. Berry’s Massage Cream
Dr. Berry’s Crème Elite
Marinello Face Cream10
Velvetina Vanishing Cream11
Woodbury’s Face Cream
Gervaise Graham Hygienic Skin Cream
E. Burnham Kalos Skin Rejuvenator
Black-and-white newspaper, catalog and magazine etchings make it difficult to determine the kind of glass used for these product jars. In general, however, the products all appear to have been packaged in “opal” or milk glass, a white glass meant to invoke the appearance of china or porcelain and compounded with a variety of recipes. The product jars we have found, all in white glass, tend to confirm the notion that white glass predominated in all of the products in which this style of jar was sold.
1. How were 2-9-S-1 siblings found?
Dr. Berry’s Freckle Ointment was the first product identified as having been contained in the same style jar as 2-9-S-1. It was located for sale on an Etsy.com auction in late 2010 after a survey of thousands of images on the internet, using key words such as “Hazel-Atlas jar” and “Hazel-Atlas ointment,” or simply “Glass Jar.”
The other Dr. Berry’s products, Dr. Berry’s Massage Cream and Dr. Berry’s Crème Elite, were identified from advertisements in druggists’ trade journals on Google Books. These appear far less often in the trade journals than does Dr. Berry’s Freckle Ointment. Gervaise Graham Hygienic Skin Cream and E. Burnham Kalos Skin Rejuvenator, and additional advertisements for Dr. Berry’s Freckle Ointment, were found in the Sears, Roebuck and Co. catalogs issued from 1908 to 1933. The remainder of these products, Marinello Face Cream, Velvetina Vanishing Cream, and Woodbury’s Face Cream were found through a search of online auctions.
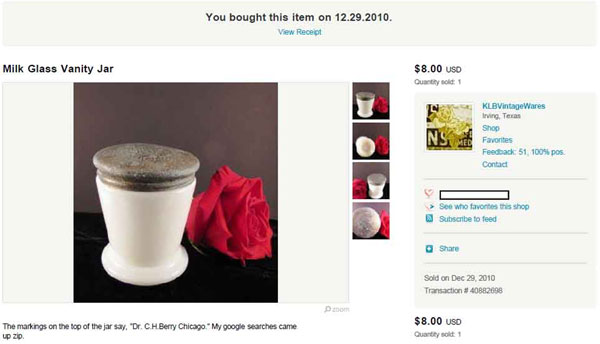
Figure 9. The first jar located in the same style as 2-9-S-1.
2. What general types of products did these jars contain?
 |
| Figure 10. Side label of Dr. Berry’s Freckle Ointment. TIGHAR photo. |
All of these products may be classified as women’s cosmetics, but they seem also to have been used as patent medicines for keeping a good complexion. Dr. Berry’s Freckle Ointment, for example, in addition to its presentation in advertisements as a cosmetic, was also frequently marketed as a patent medicine, “a medicated freckle ointment, made along scientific lines” for “eczema, pimples, freckles, tan, moth patches, muddy complexion and all discolorations of the skin.”
An employee has indicated, in a history he wrote of his company, that Hazel-Atlas jars were sold to manufacturers of a far greater array of products than just patent medicines and cosmetics. These included inks, glues, pastes and shoe polishes, in addition to foods such as jams, mayonnaise, ripe olives, and chipped beef.12 Despite the diversity of Hazel-Atlas’ lines of business, the only types of products for which we have located jars in the same style as 2-9-S-1 are for patent medicines and cosmetics.
The fact that Sears, Roebuck and Co. frequently exhibited these jars in the cosmetics section of its catalogs from 1908 to 1933 reinforces 2-9-S-1’s apparent association with these distinct types of products. Several of the products on the above list appear in its pages, under headings such as “Dainty Toilet Creams and Lotions” (1915 catalog); “Toilet and Beauty Products” (1908 catalog); “Face Creams of Quality” (1911 catalog); and “Freckle and Bleach Creams” (1930 catalog).
After 1933, however, all of the jars in this style abruptly vanish from the catalog. Most of the products continued to be packaged in pillbox styled round jars with more modern labels, replacing the illustration of a Victorian-era woman’s face with a more stylized female. None of these products continued in the catalog in any format after 1953. (Click here to see a database on ointment jars exhibited in the Sears, Roebuck and Co. catalogs.)
 |
 |
| Figure 11. Old and new styles of Dr. Berry’s Freckle Ointment. The old style, sold in the Sears, Roebuck and Co. catalog from 1908 to 1933, is on the left (TIGHAR photo). The pillbox style, offered for sale in the same catalog after 1933, is on the right (TIGHAR photo by Joe Cerniglia). |
|
| Searching for Siblings: Comparative Studies |
1. Comparison by Size
All of the jars we have found on the antique market in the style of 2-9-S-1 are either slightly smaller or slightly larger than 2-9-S-1, with one exception. We have located a clear transparent jar (which we have named as the “clear facsimile”) in the same size and style as 2-9-S-1; however, since it is unlabeled, we do not know which product, if any, it once contained.
We have an empty box labeled as Dr. Berry’s Freckle Ointment in which 2-9-S-1 fits perfectly; therefore, we know that Dr. Berry’s Freckle Ointment at some point was manufactured in the same size jar as 2-9-S-1.13
 |
 |
| Figure 12. A clear facsimile jar in the same size and style as 2-9-S-1. TIGHAR photo. |
 |
 |
| Figure 13. The photo on the left shows 2-9-S-1 fitting perfectly inside a box for Dr. Berry’s Freckle Ointment. The photo on the right shows an unsuccessful attempt to put a white jar of Dr. Berry’s Freckle Ointment in the same box. TIGHAR photo. |
2. Comparison by Thickness
The thickness of 2-9-S-1 is just under 7 mm at its rim. A verified jar of Dr. Berry’s Freckle Ointment that we have obtained on eBay is about 5 mm in thickness at its rim.
3. Comparison by Weight
2-9-S-1 weighs 109 grams; however, we have about 80% of its original material. We would estimate the original jar would have weighed about 131 grams. The clear facsimile jar in the same size and style as 2-9-S-1 weighs 109 grams, which is 18% less than the putative weight of 2-9-S-1.
4. Comparison by Color
All the labeled sibling jars we have found are white (opal) glass. We have not found any labeled product jars, in any style, with the same type of colorless, translucent glass that is uniquely characteristic of 2-9-S-1. The clear facsimile jar, shown in Figure 12 above, is colorless, like 2-9-S-1, but it is also completely transparent. 2-9-S-1, by comparison, is translucent; that is, it is neither fully opaque nor fully transparent.
5. Comparison by Logo
The clear facsimile jar, and many, but not all, of the white jars we have located, including some white jars for Dr. C.H. Berry’s Freckle Ointment, have the Hazel-Atlas logo embossed on the center of their bases. The logo, H over A, matches the one found on 2-9-S-1.
|
| What Did 2-9-S-1 Contain? |
1. Surface Analysis of 2-9-S-1
 |
| Figure 14. Faint whitish haze visible on surface of 2-9-S-1. TIGHAR photo by Joe Cerniglia. |
A base shard from 2-9-S-1 had deposited on its interior cavity an extremely faint whitish haze, possibly a remnant from its original contents. Dr. Geoffrey Cunnar, in his assessment of use wear patterns on other shards of 2-9-S-1, also noted an “attached residue,” but it is unclear whether he was referring to accretions from the natural environment or what he believed to be remnants of 2-9-S-1’s original contents. The remnant we observed appeared from later chemical testing to be the latter.
 The surface of the interior cavity of this base shard from 2-9-S-1 was leached with acid and tested using a very sensitive spectroscopic technique (ICP-MS) for the presence and amount of 69 elements. The exterior of the same shard was also tested using the same method. The test results showed mercury in the interior at a level of 4 micrograms (µg) per liter (L). The exterior showed no detectable mercury. (See Jar Shard Report.) The surface of the interior cavity of this base shard from 2-9-S-1 was leached with acid and tested using a very sensitive spectroscopic technique (ICP-MS) for the presence and amount of 69 elements. The exterior of the same shard was also tested using the same method. The test results showed mercury in the interior at a level of 4 micrograms (µg) per liter (L). The exterior showed no detectable mercury. (See Jar Shard Report.)
Mercury was a commonly used ingredient in commercial freckle and fading ointments of the early 20th century, such as Dr. Berry’s Freckle Ointment. In the context of an ointment in this style of jar, it had but a single purpose, which was to lighten the skin and to render its appearance more uniformly toned and free of blemishes. It accomplished this by inhibiting the production of melanin.14 Government agencies and professionals, who realized mercury’s toxic effects, deplored its use, but the ointments were nevertheless popular with women.

Figure 15. 1912 Injunction against Berry’s Freckle Ointment.
 While the level of mercury detected in the interior base cavity is very small, it is significant when compared with the size of the surface area on which it was detected, an area not much larger than an average postage stamp. Officials from the New Jersey Department of Health and Senior Services have said in a report that “levels around or below 1 microgram (µg) per 100 square centimeters of surface area” can be considered “clean.”15 (See Controlling Metallic Mercury in the Workplace.) While the level of mercury detected in the interior base cavity is very small, it is significant when compared with the size of the surface area on which it was detected, an area not much larger than an average postage stamp. Officials from the New Jersey Department of Health and Senior Services have said in a report that “levels around or below 1 microgram (µg) per 100 square centimeters of surface area” can be considered “clean.”15 (See Controlling Metallic Mercury in the Workplace.)
We calculated mercury levels on the shard at 2.9 times a clean background, based upon an estimate of how much surface area was actually tested. We are confident that this is a reasonably accurate estimate of the surface contamination on the shard.16 (Click here for a spreadsheet detailing mercury level comparisons.)
2. Could the Mercury Have Adhered to the Glass from the Environment?
A review of the properties of ammoniated mercury, often used in freckle preparations of the early 20th century, suggests that it is refractory, relatively insoluble, and has a high vaporization temperature, unlike metallic mercury or its oxides, which are found at low levels in the environment as a result of pollution from coal plants or wind-driven aerosols. Ammoniated mercury, as found in freckle ointments, would readily and durably adhere to glass.
Metallic mercury from the environment, by contrast, does not adhere to glass. Instead, it is washed out by rain from inert surfaces such as glass into soil. If mercury had deposited, however temporarily, on the glass due to environmental contamination, it would have been found on both the interior and exterior surfaces of 2-9-S-1. We would expect many other heavy metals such as lead along with the mercury to have been found on both the interior and the exterior of the glass if the source of the mercury had been an environmental one, such as from coal plants or volcanic ash deposition. Both the interior and exterior surfaces of 2-9-S-1 were tested, but only the interior surface showed these elements.
The mobility of mercury in the environment is biologically mediated. Most commonly, it is methylated by soil bacteria. Mercury in the environment is not associated with chemically inert surfaces. It cannot exchange from the particles on which it travels to surfaces without biological activity, and glass surfaces have no biological activity.
There were excellent scientific reasons for why environmental mercury would have been an unlikely source of the mercury found on the glass of 2-9-S-1. Still, an environmental source was not impossible and this possibility needed to be tested.
3. Did Other Glass from the Seven Site Have Adhering Mercury?
If the mercury on 2-9-S-1 arrived there from the environment, its presence would be detectable on other glass, which was not part of 2-9-S-1 but was also found at the Seven Site. If, on the other hand, mercury was not found on other glass at the Seven Site, this result would support the idea that the environment was not the source of the mercury on 2-9-S-1, but rather that its source was the product the jar once contained.
 |
| Figure 16. 2-9-S-55: A World War II-vintage Coca-Cola™ bottle fragment. TIGHAR photo. |
We tested 2-8-S-55, a neck fragment from a World War II vintage Coca-Cola bottle, most likely associated with the U.S. Coast Guard’s occasional visits to the site from 1944 to 1946. The Coca-Cola bottle was found about 8 meters west of the spot where most of the fragments of 2-9-S-1 were recovered. A laboratory tested for the presence of mercury on both the Coca-Cola bottle’s exterior and interior surfaces. No mercury was detected at the test instrument’s limit of detection.17 (See report.)

|
| Additional Control Experiments |
1. Did a Jar of Dr. Berry’s Freckle Ointment Have Mercury Adhering to its Glass?
Realizing that additional context from typical, similar and known items was essential for a valid scientific result, we wondered whether a verified, fully filled jar of Dr. Berry’s Freckle Ointment, purchased on eBay, would have detectable levels of mercury adhering to its glass.
 After removing the freckle ointment from the verified Dr. Berry’s Freckle Ointment jar and rinsing it three times with deionized water, mercury still adhered to the interior cavity. The exterior surface had no measurable level of mercury. (See Lab Report.) After removing the freckle ointment from the verified Dr. Berry’s Freckle Ointment jar and rinsing it three times with deionized water, mercury still adhered to the interior cavity. The exterior surface had no measurable level of mercury. (See Lab Report.)
Measured at 120 mg per liter (L), the mercury level on the verified Dr. Berry’s Freckle Ointment jar was 30,000 times higher than the level found on 2-9-S-1. However, we would expect a piece of glass left in the open on Nikumaroro for decades to have received far more effective rinsing and scrubbing from rain, wind-driven sand and microbial activity than anything we could simulate in a lab. Thus, we would expect mercury levels on 2-9-S-1 to be low, but still detectable and significant. We knew of no practical way to reproduce Nikumaroro’s conditions or the duration of those conditions on the verified Berry jar.
Nevertheless, the finding was significant in that it showed that a known source of ammoniated mercury would be expected to persist on a glass surface that it has contacted, even if the original source, in this case the freckle ointment, had been removed and rinsed repeatedly from the inside. We succeeded in replicating the mercury adhesion of our original result, even though we did not succeed in replicating the scale of that result.
2. Did Dr. Berry’s Freckle Ointment Contain Mercury?
 |
| Figure 17. The label of a post-1933 jar of Dr. Berry’s Freckle Ointment lists “Ammoniated Mercury” to have been contained inside at a level of 5%. TIGHAR photo by Joe Cerniglia. |
We also tested the verified Dr. Berry’s Freckle Ointment for mercury levels. Reports published at the time this product was sold stated that the ointment contained 9.8 to 12 percent ammoniated mercury, depending on the year it was produced.18 We wondered if laboratory results would confirm this. The results showed levels significantly lower than the values reported by laboratories in the early 20th century, at 5.4% total weight, but this level is still extremely toxic. This level is, however, comparable to the level of mercury that is listed on the label of a post-1933 pillbox-styled Dr. Berry’s Freckle Ointment.
We note that there is evidence that manufacturers of skin bleaches lowered mercury levels in the face of regulatory complaints to reduce mercury exposures.
3. Did Another Ointment in the Same Style of Jar Contain Mercury?
We then wondered whether mercury in ointment jars was unique to freckle ointments, or whether it might have been contained in other ointment jars for products labeled for purposes other than the bleaching of the skin.
To answer these questions, we tested a jar that has the same style as 2-9-S-1 and contains a product other than Dr. Berry’s Freckle Ointment. (We know of other such products but, since the jar is rare, have not been able to obtain them for testing.) The product we selected for this test was Burnham Kalos Skin Rejuvenator. We have a jar of Burnham’s that is more than 60% filled with product.
 Testing of both the Burnham’s cream, and the surfaces of the glass jar in which the Burnham’s was sold, showed no detectable levels of mercury.19 (See Lab Report.) Testing of both the Burnham’s cream, and the surfaces of the glass jar in which the Burnham’s was sold, showed no detectable levels of mercury.19 (See Lab Report.)
This test suggested that mercury was probably not randomly included in the ingredients of ointments and topical creams that were not specifically marketed as bleaching ointments. On the contrary, wherever mercury existed in cosmetics, it appears to have had a specific purpose. Richard Swiderski, an expert in the history of mercury, states that during the early years of the 20th century “mercury compounds dominated the explicitly anti-freckle applications, and no other chemistry was used in them.”20 Mercury was no accidental ingredient in the manufacture of skin products sold as cosmetics, and, one may realistically assume, it was no accidental ingredient in the former contents of 2-9-S-1.

Figure 18. A jar of E. Burnham Kalos Skin Rejuvenator next to a jar of Dr. Berry’s Freckle Ointment. TIGHAR photo by Joe Cerniglia.
| Glass Item Tested |
Artifact Number |
Where Obtained |
Mercury on Inside? |
Mercury on Outside? |
Mercury in Contents? |
Ointment Pot |
2-9-S-1 |
Seven Site on Nikumaroro |
Yes @ 4 µg/L |
No |
n/a. The jar was empty. |
World War II Coca-Cola™ bottle |
2-8-S-55 |
Seven Site on Nikumaroro |
No |
No |
n/a. The bottle was empty. |
Dr. Berry’s Freckle Ointment |
None |
eBay |
Yes @ 120 mg/L |
No |
Yes @ 5.4% weight |
Burnham Kalos Skin Rejuvenator |
None |
eBay |
No |
No |
No |
Summary of the results of surface analyses of 2-9-S-1, 2-8-S-55 (the Coca-Cola™ bottle) and two additional product jars purchased on eBay.
|
| Did 2-9-S-1 Contain Dr. Berry’s Freckle Ointment? |
We appear to have a jar that may have been manufactured in the 1930s, or later, which contained a mercury-bearing substance, in the same style as Dr. Berry’s Freckle Ointment, but did 2-9-S-1 contain Dr. Berry’s Freckle Ointment? Linking 2-9-S-1 definitely to this particular product is far from certain. All of the Dr. Berry’s Freckle Ointment jars we have seen are white. 2-9-S-1 is colorless, translucent glass. If Dr. Berry’s was ever produced and sold in colorless format, we are not aware of it.21 If freckle ointments other than those made by the Dr. C.H. Berry Company sold in colorless versions of this style of jar, we have not seen them.
Reconciling the paradox of 2-9-S-1’s appearance with known examples would appear to be difficult. One may, however, envision a scenario for 2-9-S-1’s manufacture that does not necessarily include the white jars of the Dr. C.H. Berry Company, but does include freckle ointment. One such scenario is the possibility of a freckle or tan-fighting ointment from a pharmacy. As William Kelly relates in Prescribed Medications and the Public Health, “In the 1930s and 1940s, nearly 60 percent of all prescriptions were compounded – made from scratch – by pharmacists using various chemicals and medicaments... The products compounded... include topical creams and ointments.”22
The Hazel-Atlas Glass Company, manufacturer of 2-9-S-1, may also contain within its history a possible rationale for how a freckle ointment could appear in a different color jar than the one in which freckle ointments were traditionally sold. According to an employee of the company, John S. Algeo, Hazel-Atlas did not begin as a producer of cosmetic jars. Algeo says: “Up to that time (1914) our volume was in the medicinal field.” He adds, “We made cosmetics but the proportion was small and cosmetic items were listed in our catalog and literature as ‘Ointments’ or ‘Druggist Specialties.’” The market for glassware in which to sell cosmetics only gradually expanded after World War I. It was not until circa 1930, according to Algeo, that “we dignified that line by separating it in our literature from the ‘Ointment’ or ‘Druggist’ line and using the name ‘Cosmetic’.”23 Since the cosmetic jars of the Hazel-Atlas Company traced their lineage back to this druggist line that Algeo describes, it is not unreasonable to suppose that some jars from these parallel lines might have shared parallel styles and features, though perhaps not all styles and features. Varying the color of the jars may have been a way to distinguish glass products from these lines.
The decision of Hazel-Atlas to separate its cosmetics from its medicinal lines may have been influenced by the evolution and marketing decisions of such companies as Dr. C.H. Berry. The C.H. Berry Company originally billed itself, not as a cosmetics firm, but as a patent medicine producer from an actual medical practice of an actual Dr. Berry in the Chicago area.24 C.H. Berry Company took its name from “The Eminent Chicago Specialist,” who practiced in the city in the late 1800s treating, among other maladies, skin diseases, and in its first incarnation called itself the “Dr. C.H. Berry Chemical Company.”
Only later in its history did the name of C.H. Berry became connected in the public mind with cosmetics for women. This is worth noting as we attempt to establish a possible connection between a quite common commercial product of the 1930s, sold in white jars, and 2-9-S-1, which is colorless. While 2-9-S-1 may or may not represent a product of the C.H. Berry Company, it is possible that it represents a freckle ointment from the older tradition, of which Dr. Berry had been a part, of privately compounded patent medicines from pharmacies, which were common in the years before mass marketing, attendant mass production, and the transformation of patent medicines into “patent cosmetics,” took hold. Indeed, the history of both the Hazel-Atlas Company and the Dr. C.H. Berry Company bears out a certain ambiguity between cosmetics and medicines in their various lines of business. Perhaps 2-9-S-1 itself, with its strange and exceedingly rare colorless format, symbolizes some of this ambiguity.
The ambiguity seems to have extended to the buying habits of female consumers as well. Kathy Peiss, who has written extensively on the history of cosmetics in the United States, often remarks upon the importance of pharmacies and druggists to the cosmetics trade for items such as creams and ointments. Speaking about the mid-19th century, Peiss wrote about the emerging tradition of women relying upon pharmacies for their cosmetics:
Women who did not make their own cosmetics had two choices. They could go to a pharmacist (or, less often, a hairdresser) who compounded preparations under a ‘house’ label, or they could purchase commercial products made by perfumers and ‘patent cosmetic’ firms. The distinction was not a trivial one, for it pitched local druggists using standard formulas against remote manufacturers of secret skin remedies and beauty aids.25
By the close of the 19th century, the custom of buying these products from pharmacies had grown even more prevalent:
Some could not afford to buy the products they desired and asked for a comparable formula to mix at home; others wanted a recipe to take to the drugstore, to be compounded and purchased there.26
By the 1930s, the era in which we believe 2-9-S-1 was manufactured, these traditions of making one’s cosmetics in the home or having a pharmacist personally compound them were clearly on the wane as mass media began to influence how and where these products were purchased.
But there is evidence that the traditions persisted and certainly did not fade away all at once, and there were advantages to them as well. For one, the practice of compounding one’s cosmetics in a pharmacy or at home was a discreet way of purchasing them. Stigmas of artifice and overzealous social climbing still clung to cosmetics, and discretion was a way of avoiding these.27 For another, taking control of the formularies of these cosmetics was a way to help ensure that they were safer. The dangers of ingredients such as mercury could be minimized to some extent if one compounded the products oneself, or asked a pharmacist to do this.28
We know, therefore, that cosmetics could be and often were privately compounded in pharmacies or in the home; we can only speculate, however, on whether this is what actually happened when 2-9-S-1 was purchased and whether this somehow relates to the fact that the jar is of a different color than would have been found in a commercial product. The answer to this question remains beyond the reach of a laboratory test.
|
| Did Amelia Earhart Use Freckle Ointment? |
Earhart’s concern for her appearance, while reasonably well documented and remarked upon by biographers, is difficult to verify, especially in terms of whether that concern included a particular dislike of freckles. With the exception of one unscripted and highly uncharacteristic remark in 1928, she is not reported to have discussed her attitude toward freckles publicly during her life, except perhaps in euphemism as a problem with “severe sunburn,” which can exacerbate freckles. Earhart rarely revealed personal biographical details in casual encounters with friends or the press.
The obscurity of the details notwithstanding, there are a number of instances in which Amelia Earhart or her close friends and relatives left clues pointing to the possibility that she did indeed worry about her complexion, and did seek a remedy for the worry in the form of a “tallow preparation.” The best examples we have found of these clues are as follows:
| 1 |
From p. 164, Courage is the Price, 1963, by Muriel Earhart Morrissey (Amelia Earhart’s sister). This refers to the 1928 New York ticker tape parade after the Friendship flight:
“Mother, Miss Perkins, and I had a few minutes with Amelia when her plane from New York landed at the unfinished Boston Airport. Soon Amelia was called to take her place with the two men for the ride to City Hall. She stood for a moment on the steps for the photographers; then, as she started to get into the waiting limousine, several voices called, ‘Take off your hat, Amelia! Take off your hat!’ for her windswept short hair was a novelty to many.
“Amelia paused, and with a slight grimace pulled off her hat, a light cloche of straw with a small brim, and tossed it to me, saying as she did so, ‘Here’s where I get sixty more freckles on my poor nose, I guess!’”
|
| 2 |
From p. 202, Courage is the Price, 1963, by Muriel Earhart Morrissey. “For two years Mother kept a suitcase packed with a few simple clothes, cold cream for sunburn, and scissors to cut her hair, in case Amelia were discovered on a tropical island.” |
| 3 |
From p. 94, The Fun of It, 1932, by Amelia Earhart. “Flying so much had caused a severe sunburn. For most of the journey, I wore a close fitting hat instead of the helmet which left a sunburned streak across my cheeks. Goggles cannot be abandoned on long hops, except in closed ships. They, of course, bequeath unburned rings of white around the eyes. In my log book, I noted that when and if I reached Los Angeles, I should resemble a horned toad.” |
| 4 |
From the Omaha Morning World-Herald, October 11, 1928, in which Earhart asked for advice from actress Eve Casanova on her “weather-beaten appearance” from sun exposure, of which she was “ashamed.” “‘How do you prevent sunburn and keep that lovely complexion?’ Miss Earhart wanted to know. ‘I get so burned and tanned that I’m sometimes ashamed of my weather-beaten appearance.’” (See article.) |
 |
| 5 |
 From the Abilene (Texas) Reporter-News, July 11, 1937, a retrospective on Earhart’s life and earlier interactions with the townsfolk during a cross-country autogiro tour in June of 1931: “Next morning, she was quiet, praised the tallow preparation I had given her for her sunburn, talked with George Paxton, Jr., and Mr. Oldham’s father and posed for some Kodak pictures.” (See article.) From the Abilene (Texas) Reporter-News, July 11, 1937, a retrospective on Earhart’s life and earlier interactions with the townsfolk during a cross-country autogiro tour in June of 1931: “Next morning, she was quiet, praised the tallow preparation I had given her for her sunburn, talked with George Paxton, Jr., and Mr. Oldham’s father and posed for some Kodak pictures.” (See article.) |
As tantalizing as these quotations appear to be, we have found nothing by way of a document or a photograph that would conclusively establish that Amelia Earhart used freckle ointment or brought freckle ointment on any of her flights. Even if we were to find such documentation, this would still not prove that Earhart brought 2-9-S-1 to the Seven Site on Nikumaroro Island.
|
| Conclusions |
1. Who brought 2-9-S-1 to the Seven Site?
If one removes Earhart as the source of a freckle ointment, as 2-9-S-1 appears to be given its mercuric residue, then we are left still with the fact that this artifact was found at the Seven Site, alongside other artifacts suggesting lotions, skin unguents, and cosmetics. Someone brought 2-9-S-1 to the location where it was found. If Earhart did not bring 2-9-S-1 to the Seven Site, who did bring it?
We have found no evidence that known residents of the island used freckle ointment. Tuvaluan and i-Kiribati colonists seem unlikely to have had such an item in their possession, although it certainly would not have been impossible. In all of the archival research conducted by TIGHAR, no evidence has ever emerged that Pacific Islanders of Nikumaroro engaged in skin-bleaching practices or used such products for these practices.
2-9-S-1 seems a less than likely choice for a U.S. Coast Guardsman from the Coast Guard LORAN station, active from 1944 to 1946, or a British overseer of the Nikumaroro colony, active from 1938 to 1963. The design of 2-9-S-1, which is tapered with flowing art deco lines, is very feminine in appearance and not likely to have attracted a male clientele. All of the products located in the same style as 2-9-S-1 were marketed to women.
Paul Laxton, officer-in-charge of Nikumaroro from 1949, had a wife who could have visited the site, but Mrs. Laxton was from Britain and lived on Nikumaroro in the late 1940s and early 1950s. Many of the Seven Site artifacts, including 2-9-S-1, seem to be of mid-1930s U.S. manufacture. Mrs. Laxton also lived in the Rest House in the colonial village at Ritiati, at the opposite end of the island and across the lagoon from the Seven Site.
2-9-S-1 might also be linked with the castaway, who may have been Earhart, and who appears to have deposited sometime prior to 1940 (see Bones Chronology) in the same vicinity in which 2-9-S-1 was found, several items including:
| » |
Enough bird and fish bones to have provided food upon which one could subsist for days or weeks (Birds); |
| » |
A bottle of Campana Italian Balm (Lotion Bottle); |
| » |
  Two bottles, a pre-World War II beer bottle and a bottle of St. Joseph Liniment, whose bottoms appear burned in a fire, perhaps in an attempt to purify water; Two bottles, a pre-World War II beer bottle and a bottle of St. Joseph Liniment, whose bottoms appear burned in a fire, perhaps in an attempt to purify water; |
| » |
 A utility knife with the blades missing (although this could be from the U.S. Coast Guard); A utility knife with the blades missing (although this could be from the U.S. Coast Guard); |
| » |
The possible remains of a woman’s compact; |
| » |
A box for a sextant, and; |
| » |
Thirteen human bones, including a skull, apparently associated with a female of northern European ancestry. |
2. Summary of Findings in Context
We have an ointment pot (2-9-S-1) found at the Seven Site on Nikumaroro Island whose style was almost certainly used for patent medicines and/or cosmetics. The history and the use of patent medicines and cosmetics overlapped significantly in the early years of the 20th century.
Ointments need protection from the ultraviolet spectrum of sunlight. White milk glass jars offered this protection by the fact that they were opaque. 2-9-S-1 has translucent glass, but the barium content in its glass formula endowed it with a high refractive index. This highly refractive quality would give the contents of 2-9-S-1 a level of protection from ultraviolet rays comparable to that afforded by white milk glass.
The earliest date of use of the manufacturer’s logo on the base of 2-9-S-1 (1923), the appearance of 2-9-S-1 in Sears, Roebuck and Co. catalogs (1908-1933), and the date on a unique Hazel-Atlas patent for the chemistry of the constituent elements (1936) in the glass of 2-9-S-1 seem to indicate 2-9-S-1 was made more probably in the 1930s than in the 1920s. It could not have been made earlier than 1923 because the logo would have been absent from jars from that period. It could have been made after the 1930s, but advertisements from the Sears, Roebuck and Co. catalog would seem to indicate 2-9-S-1 was already outdated by the mid-1930s.
2-9-S-1 was almost certainly manufactured in the U.S. for a product marketed to women. None of the products found in this style of jar appear to have been marketed to men.
2-9-S-1 contained mercury. The mercury did not come from the environment; it came from a substance that 2-9-S-1 once contained.
Mercury in ointments in this style of jar had a single purpose, which was to lighten the skin. 2-9-S-1 therefore most likely did contain a freckle ointment, or a preparation, regardless of what it may have been called, whose function would have been indistinguishable from freckle ointment.
The only commercial product we have found in this style of jar that also contained mercury was Dr. Berry’s Freckle Ointment.
No jar in the style of 2-9-S-1 has been found with the same colorless, translucent glass, leading us to speculate that a privately compounded freckle ointment, perhaps from a drug store, may have once been contained inside. This speculation would not rule out other possibilities. There may have been a colorless jar of Dr. Berry’s Freckle Ointment that we have not yet seen.
Amelia Earhart was concerned about her overall appearance, what the sun was doing to her skin, and her freckles, all three of which were probably interrelated. She is known to have sought advice from strangers in alleviating these concerns and even accepted a “tallow preparation” for sunburn from an Abilene, Texas family with whom she visited in 1931.
We have not found an exact twin to 2-9-S-1, but the search continues.
|
|


