 |
A detailed theoretical explanation of the treatment of composite objects made up of aluminium and iron alloy have been discussed in detail elsewhere.1 However it is necessary to outline some basic theory behind the treatment plan.
Necessity of the treatment.
Most submarine artefacts made of aluminium alloys are also what is termed “composites,” that is they often contain large percentages of more noble metals such as iron and copper and non-conductive material such as leather, wood, insulating fabrics, etc. During the period of immersion, intensive contact corrosion occurs between the metals at the expense of the aluminium alloys. These corrosion reactions are irreversible.
During immersion, the rate of corrosion is slow but this increases with exposure to the atmosphere. The study of corrosion forms, particularly in the corrosion layer covering the degraded alloy’s surface, has demonstrated that chloride species are present at the very tips of the corrosion pits (formed during the immersion) and are responsible for continued degradation. Further, the very presence of corrosion products may accelerate this process.
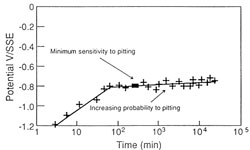
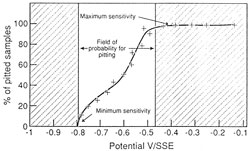
The immersion of an object in an aqueous media (whether having the ability to complex the metallic species contained in the corrosion products or not) designed to remove chlorides is a dangerous procedure. It is possible that the dissolved chloride concentration will increase to a point where they will re-attack the metal surface. This phenomenon is due to an increase in the corrosion potential of the alloys during immersion (fig. 11.). After a certain time the area in which the alloy is sensitive to pitting corrosion is entered (fig. 12.)
| Figure 11. Evolution of Ecorr as a function of time for aluminium-magnesium-silicon (6082) alloy in sodium citrate solution, pH=5.4 [NaCl]=10-3M, T=10°C. | Figure 12. Determination of the field of probability of pitting corrosion of 6082 alloy (Al-Mg-Si), determined from a series of smaples subjected to anodic polarization, pH=5.4, [NaCl]=10-3M, T=10°C. |
 |
 |
Treatment conditions.
A cathodic polarization avoids this drawback. However, it is important to be aware that in the pH range of between 4 and 9, where the surface is normally protected, aluminium alloys are subject to cathodic corrosion due to a decrease in the isolating properties of the superficial oxide film. Depending on the conditions, this can take the appearance of pitting or a more generalised corrosion.
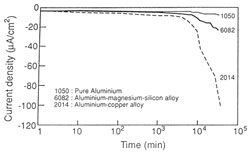
To avoid the risk of this corrosion due to localised “alkalisation” of the solution close to the metallic surface, the polarization is conducted in a slightly acid and buffered solution of sodium citrate (pH = 5.4). The treatment is undertaken using potentiometric conditions which manifest in two stages as the polarization preceeds (fig. 13).
 |
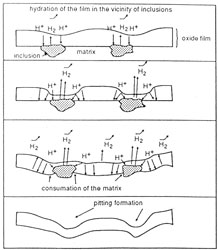 |
| Figure 13. Cathodic polarization at -1.4V(SSE) for various aluminium alloys in a sodium citrate solution, pH=5.4, without agitation and deaeration. [NaCl]=10-3M | Figure 14. Mechanism for cathodic corrosion of aluminium alloys in a buffered solution. |
The polarization currents begin at a low value, particularly for noncorroded alloys. This corresponds to the hydration of the initial oxide film after the incorporation of H+ ions from the media. This is more prevalent near inclusions containing more noble elements such as copper and iron which can be present in the aluminium alloy. The localised modification of the film now allows corrosion close to these inclusions. The surrounding matrix is dissolved and eventually the inclusions are removed (fig. 14).
The extent to which this process occurs is controlled by such factors as the type of alloy, the aeration of the media and the cathodic potential. Thus the increase in current densities is of importance.
After several days of polarization, pits appear on the surface either in groups or in isolation. Corrosion is particularly prevalent when hydrogen bubbles, which form on the surface of the metal, are not removed, since localised alkalisation can occur under them. Efficient stirring of the solution minimises this problem.
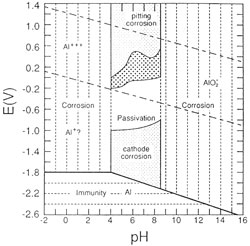 |
| Figure 15. Experimental pH-potential diagram showing the sensitivity of 6082 alloy to cathodic corrosion. |
Thus an experimental Potential – pH diagram can now be constructed, modified to the required conditions. A “protection” area now appears in the region which is suitable for dechlorination treatments. (fig. 15).
It was anticipated that the treatment procedure would generally follow that which has already been adopted in Australia for the treatment of the Pratt and Whitney “Twin Wasp” engine.
The BMW engine, having been rescued from a freshwater environment, had the appearance of being in a far less corroded condition when compared to the Pratt and Whitney, which was recovered from sea-water and had been left exposed to the atmosphere untreated for some 20 years. Obvious signs of corrosion due to chloride contamination were absent on the BMW engine itself, although some of the smaller parts which were salvaged at the same time manifested typically blue/green colouration of Al/Cu alloy corrosion products. A thin layer of corrosion products and concretions were apparent on both iron and aluminium components (fig. 16.).
| Figure 16. A thin layer of corrosion products and concretions cover both iron and aluminium components. | |
 |
 |
Notwithstanding, a decision was made to conduct the treatment according to the following global plan:
1. Pre-cleaning.
The employment of various mechanical methods to remove dirt, loose concretions and corrosion products before treatment begins.
2. Pre-treatment.
Immersion of the engine in a solution of citric acid buffered to a pH of 5.4 with sodium hydroxide. This is designed to remove concretions and unstable corrosion products perhaps containing iron species. If present, superficial chloride species will also be extracted at this time.
3. Removal of iron concretions using cathodic polarization.
The loosening and removal of concretions on the iron parts is the objective here. This can be facilitated by agitation and the controlled generation of hydrogen bubbles at the metal – concretion interface.
As is now established, the iron components of any composite object are treated first, before that of the aluminium alloy. A basic solution is chosen for the treatment. In this way, any failure of the cathodic potential will result only in the formation of a passive oxide film on the surface of the aluminium alloy and thus protect it. Sodium metasilicate is chosen in particular as it has been shown2 to be an effective corrosion inhibitor for aluminium. A concentration of 0.04M was selected as being the most efficient concentration for the protection of a corroded aluminium surface.
4. Removal of the silicates from the metal surfaces.
This is usually conducted by immersing the object in tap water utilising cathodic protection and monitoring the water for a rise in pH. This stage may be repeated as many times as deemed necessary until it is apparent that the pH is not being altered by the dissolution of silicate species.
5. Dechlorination of the object using cathodic polarization.
It is proposed that controlled cathodic polarization at a constant potential be undertaken to rapidly extract chlorides contained in aluminium alloys.
This enables the extraction of chloride ions from deep within the materials. Efficiency of this process can be increased by careful adjustment of the cathodic potential. However, as described earlier, these values are limited by the aluminium alloys sensitivity to cathodic corrosion.
The risk of hydrogen embrittlement is always present however. To guard against this the treatment is conducted at a slightly less negative potential than the theoretical limit.
The treatment is conducted in a citric acid solution buffered to a pH of 5.4 using sodium hydroxide. It is important at this stage to monitor chloride ion concentration and so deionized water must be used.
6. Citrate decontamination.
Removal of the citrate species by immersion of the object in tap water is undertaken while the object is cathodically protected. Again this step can be repeated as many times as considered necessary.
7. Finishing Treatments.
Final mechanical work and the application of protective coating to meet conservation requirements standards are now undertaken to prepare the object for museum display and/or storage.
[2] Unpublished results obtained from experimental work conducted at AWM and Valectra.
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
Copyright 2021 by TIGHAR, a non-profit foundation. No portion of the TIGHAR Website may be reproduced by xerographic, photographic, digital or any other means for any purpose. No portion of the TIGHAR Website may be stored in a retrieval system, copied, transmitted or transferred in any form or by any means, whether electronic, mechanical, digital, photographic, magnetic or otherwise, for any purpose without the express, written permission of TIGHAR. All rights reserved. Contact us at: info@tighar.org • Phone: 610.467.1937 • JOIN NOW |